Renewable energy sources such as wind and solar power are important for the sustainable energy supply to replace the fossil fuels and to mitigate climate changes. These renewable sources require storage and conversion devices to overcome their non-constant energy supply. Solid Oxide Electrolysis (SOEC) technology is an efficient method to converse the renewable electricity into chemical energy. In reverse mode, solid oxide fuel cell (SOFC) operation mode, the stored fuel can be used to regenerate the electricity. SOECs are assembled of three main components, namely, the anode (an oxygen electrode), the cathode (a fuel electrode) and the dense electrolyte. The cathode side is fed with a gas stream composed of either H2O, CO2 or their mixture, which is reduced to H2, CO or syngas fuel.
The dissociated oxygen ions are transported from the cathode side to the anode through the dense electrolyte driven by an external voltage. Finally, the oxygen ions are again oxidized to oxygen molecules in the anode. A last crucial part is the interconnect to couple multiple cells in one stack. It acts as a current collector, but also as a physical barrier separating the oxygen and hydrogen gas atmospheres between the different cells. All these active components of the SOC unit cell comprise of rare-earth elements. The cost of these RE elements not only depends on the production cost but is also heavily influenced by the industrial and economic policies of rare-earth producing countries since rare earths are considered as one of the strategic materials for clean-energy related technologies. An increase in the cost of rare-earths may endanger the commercialization of SOEC based technologies due to the higher cost and/or non-availability of rare earth compounds.
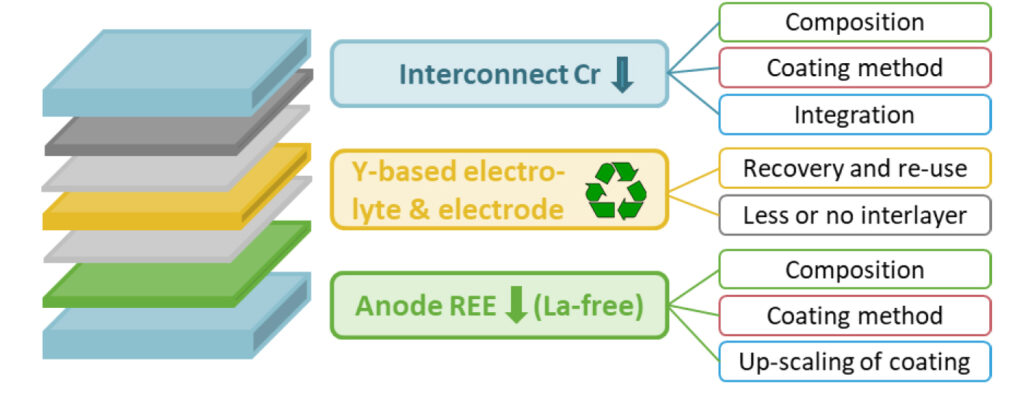
NOUVEAU will advance the development of intermediate-temperature SOEC systems with increased durability and lower REE/metal amount, by focusing on three main materials of SOEC:
i) Anode: Development of La free or reduced anode to improve stability and lifetime, recovery of
raw materials
ii) Electrolyte: Recovery and reuse of the materials, synthesis of operating electrolyte from recovered
materials
iii) Interconnect: Less Cr, in combination with protective coating
The anode and the interconnect materials are two important parts of the solid oxide cell which contain a large amount of costly metal and REE and which both suffer from degradation issues. The development of these two materials will be supported by modelling, life-cycle analysis, recovery and recyclability options. Besides the composition of these materials, the coating methodology is also essential as it determines the microstructure, porosity and thickness of the electrode and the protective coating.
By improving the coating methods, the microstructure and porosity can be fine-tuned, which can lead to a further reduction of valuable metals. One aspect that will be taken into account is the scalability of the developed coating method and coating suspensions.
The electrolyte material contains a large amount of REE, either Yttrium, Gadolinium or Cerium. An alternative REE-free electrolyte is very challenging and rarely studied in literature. Therefore, the focus will be on the recovery and recyclability of these excellent electrolyte material.